Water is a good solvent for many compounds including the gases contained in the air like oxygen and carbon dioxide.
Amount of the oxygen soluble in water depends on a number of factors: the depth of the tank, the water temperature, the presence of plants and animals. Water in motion tend to be better oxygenated than standing water. Amount of the oxygen soluble in the water is inversely proportional to the temperature of that water - higher temperature than less oxygen in the water. In the aquarium water is oxygenated mainly from the air. If it remains stationary only the upper layer gets oxygenated. Additional source of the oxygen are plants. Oxygen users are fish and other aquarium animals, micro-organisms living in the water (mainly in the ground or in the filter). Plants are also becoming oxygen users at night. Hence the need for additional water aeration at night. If the tank is well oxygenated then there are processes like aerobic decomposition of organic pollutants and oxidation of organic compounds (proteins, carbohydrates and fats) into simple compounds easily assimilable by fish and plants. Anaerobic processes (lack of oxygen in the aquarium) lead to the formation of toxic components, harmful to aquarium inhabitants substances (eg hydrogen sulfide, organic acids, polycyclic aromatic hydrocarbons), which are sometimes causing unpleasant odor.
To increase the amount of oxygen in the tank you need to install an additional filter or aerator, replace part of the water with fresh water or increase amount of the plants in the aquarium.
During the day, carbon dioxide is excreted by fish and other aquatic animals. It is also formed in the process of decomposition of organic compounds and at night is also excreted by plants. The overcrowded aquarium with small amount of plants will lead to excessed amount of this gas in the water. Also no ventilation and excessive fertilization of ground will lead to increased amount of carbon dioxide in the water. An excessive amount of plants in relation to amount of fish in the aquarium is not recommended - in day time it leads to a deficiency of carbon dioxide but at night it will lead to oxygen shortage. It is also the cause of biological decalcification of water (plants draw carbon dioxide from calcium bicarbonate and calcium carbonate). Only aquarium with the biological balance (balanced number of fish in relation to plants, optimum temperature, pH, water hardness, the amount of light regular water exchanges, appropriate filters and aerators, loose substrate) is able to function effectively for the benefit of our fish and plants. There also must be mentioned that the excess amount of the carbon dioxide may result in lowering pH. If the exaggeration of the amount of this gas being dosed into the aquarium (above 50mg/Litr) the pH may drop to levels when fish may start dying. I will cite a few examples of dependence between pH and amount of carbon dioxide dissolved in water:
| Carbonate hardness kH [° dKH] | Amount of the carbon dioxide in the water | ||
|---|---|---|---|
| over 50mg/Litr | about 30mg/litr | below 15mg/Litr | |
| 2 | pH 6.1 | pH 6.4 | pH 6.7 |
| 5 | pH 6.4 | pH 6.7 | pH 7.1 |
| 10 | pH 6.7 | pH 7.1 | pH 7.4 |
| 20 | pH 6.9 | pH 7.4 | pH 7.7 |
The most recommended dose of carbon dioxide is 20-30mg/Liter and you should keep it to maintain the correct growth of plants. It is also important that the amount of light was in the range of 0.5 W so plants could use CO2 in the process of photosynthesis.
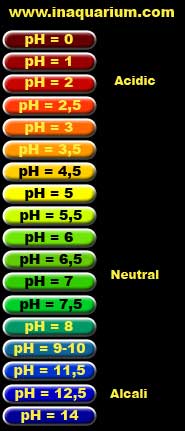
Chemical compounds dissolved in the water have influence on the water pH. In most cases water ph good for fish has a neutral pH that is pH = 7. The pH of the water can be very accurately measured using pHmeters (however this is expensive equipment to buy) or less accurately using color indicators in the form of different solutions (eg bromothymol blue - 6,2-7,6 pH range, methyl red - the range of pH 4 ,2-6, 3, but not very practical ways). It can be also measured with available at the pet stores - strip ratios (the advantage is that in addition to pH indicate other water parameters, such as: the level of carbon dioxide, water hardness, nitrites, nitrates, etc.) or ph paper strips (cellulose impregnated with litmus or other dye). In real live we ususally acidified tap water (required for the reproduction of some species) than we alkaline it. Lowering the pH can be achieved by passing it through a filter filled with peat or alder cones (lowering pH to 6-6.5 - you should keep in mind that the filtered water must be softened before). There are ready made resources in the pet shops for water acidification. To raise the pH of the water you can use ready-made preparations available in aquarium shops or sodium carbonate. To decrease the pH of the water may be mixed with distilled water or water from reverse osmosis filter gives a soft water as the result.
You can also use the dried black alder cones, which prepare a brew (pour boiling water and keep covered until cool), or use as a refill for the internal or external filter. It's hard to determine the right amount so you should carefully keep an eye on the pH value of the water. Usually 8-13 cones is used per every 100L of water in the aquarium.
Do not change the water parameters if there are fish in it. Remove fish from the aquarium first. It is also important not to exceed recommended pH value. It is always better to change the pH too little than too much. Sudden changes in water parameters including pH may harm aquarium inhabitants. That is why we change water parameters in stages.
As we know carbonate hardness (transient) KH can be removed by boil the water. Sulphates of calcium and magnesium (hardness imperishable) can be removed using suitable ion exchangers. The sum of both is called total water hardness GH which expresses in German degrees (one step corresponds to 10 mg of calcium oxide CaO or magnesium oxide MgO contained in 1 litre of water).
| Scale for water | Total water hardness GH [º German] |
|---|---|
| Very soft | 0-5 |
| Soft | 5-10 |
| Medium hard | 10-20 |
| Hard | 20-30 |
| Very Hard | over 30 |
Ion exchange (ion exchangers) remove minerals (cations and anions) from water. There are widely used cation resins for example porous material or gel which has acidic properties. When these properties are activated they takes a negative charge and are able to bind cations, in this case, calcium and magnesium). Each Ion-exchange resin has a specific capacity (the amount of ions adsorbed on their surface) and after depletion must be regenerated (Ion-exchange resin are regenerated using Sodium chloride NaCl). Exchangers work effectively with the free flow of water and must be kept under water. The pet stores are also equipped with ready-made solutions to soften water. Water hardness can be determined by laboratory methods or testers available in pet stores.
These compounds are harmful to fish. You have to strictly control the levels of these compounds. Specially harmful are nitrites and ammonia are broken down by nitrifying bacteria in the filter to nitrates which are used by plants during photosynthesis. These compounds are derived from food debris, dead plant parts and are put into an aquarium with fish feces. Ammonium compounds cause an increase in pH. The excess ammonia can stop plant growth.
Excess nitrogen compounds is caused by overpopulated aquarium environment, the accumulation of excess sludge, fish waste, decaying plant debris and food. Lack of regular water exchanges or faulty aquarium filtration also affect the increase in the concentration of nitrogen in the water.
To prevent this you need to regularly exchange part of the water (every 2 weeks about 25% water), feed the fish with smaller portions (so food is not sloped to the bottom and rot), clean the filters regularly (preferably with substitutions, water, and rinse with the water removed from the aquarium not to damage the bacterial flora supporting the filtration process). Never wash filter in the tap water and do not overpopulate aquarium (1cm of fish = 1 litre of water). You can also increase amount of plants that will use nitrates for photosynthesis effectively reducing their amount in the water.
Chlorine is a compound used in the preparation of drinking water (tap water). It is used to kill bacteria. It is also used for disinfection of water in swimming pools. As we know excess chlorine in the pool can burn human skin. The same thing happens with the fish if we will put them into the aquarium with water straight from the tap (tap water should wait for few hours). You can examine the amount of chlorine using testers available in stores. To remove it from the water you can use ready-made preparations or just leave it for few hours to let the chlorine disappear.
Arises in overpopulated tanks or when fertilizers containing phosphorus are used. Phosphates increase the amount of algae in the aquarium. The amount of these compounds can be checked using test kits available in stores. Increase the amount you can use KH2 PO4 or by giving fish much more live or frozen food. To reduce the amount of water changes, or use potassium nitrate KNO3.
Both excess and deficiency are harmful. Excess harms fish and some plants. Shortage of iron causes yellowing of the plants leaves. To increase the level of iron in the water you can use Chelate Fe. To reduce amount of iron you need to exchange part of the water.
Below is a list of acceptable and dangerous amounts of the compounds described above.
| Name | Recommended amount [mg / litre] | Dangerous amount [mg / litre] |
|---|---|---|
| Carbon dioxide CO2 | 10-15 | over 50 |
| Oxygen 0 2 | 5 | below 2 |
| Ammonia NH3 | 0 | over 1 |
| Nitrate NO 3 | 5-10 | over 50 |
| Nitrite NO2 | 0 | above 0.2 |
| Phosphates PO4 | 0.5 | above 3-4 - may increase algae growth |
| Iron | 0.1 | above 1.5-2 |