The beautiful appearance of a planted aquarium largely depends on additional fertilization of the plants it contains. And just like for humans, the best effects are brought by a balanced diet, similarly in the case of plants their fertilization should be balanced.
Plants absorb various nutrients with different intensity and speed. Contrary to appearances, this does not only depend on the given species, but also is associated with, for example, the growth stage (young plants need more nutrients) or the transition of the plant to the generative period (blooming and producing seeds).
Optimizing fertilization (adding fertilizers "just right", neither too much nor too little) helps to keep plants in excellent condition, and at the same time, it will not strain our budget too much.
There is one rule – more fertilizer does not mean better and faster growth of plants. Scientific research has proven that plants have the ability to absorb only a certain level of individual nutrients. It means that above these values we will not "improve" anything in plants, but we can only harm the aquatic environment. Dosing the sufficient amount of nutrients should be the goal of every aquarist who has plants in his tank.
There are two main methods of plant fertilization in the aquarium: PMDD and EI, which I will try to explain in the next part of this article.
This is a method of fertilizing aquarium plants with all necessary nutrients, but with limited amount of phosphorus, aimed at controlling the amount of algae in the aquarium.
This method was developed in 1996, and its creators are Paul Sears and Kevin Conlin. Initially, the method assumed that phosphates would not be dosed at all (their dosing was completely eliminated), as they are available to plants from the metabolic processes of fish, and their excess affects the unwanted growth of algae. Over time, however, it turned out to be erroneous thinking, especially in the case of aquariums with a large number of plants (planted aquarium, Dutch aquarium, natural aquarium). Then the creators of the method concluded that phosphates must also be dosed, but still in a limited amount.
There are several modifications/variations of this method, which differ in the dosing of individual components and the fertilizer itself. A more popular version of this method was once PPS-Pro (Perpetual Preservation System) and PPP Classic.
| Compound name | Dose |
|---|---|
| potassium nitrate | 1 teaspoon |
| potassium sulfate | 1 teaspoon |
| magnesium sulfate | 2.5 tablespoons |
| chelate mix: 7% Fe; 1.3% B; 2% Mn; 0.06% Mo; 0.4% Zn; 0.1% Cu; EDTA; DTPA | 1 tablespoon |
| distilled water | 300 ml |
| Compound name | Dosage |
|---|---|
| potassium nitrate | 25 [g] |
| monopotassium phosphate | 5,8 [g] |
| potassium sulfate | 11 [g] |
| magnesium sulfate heptahydrate (epsom salt) | 20 [g] |
| warm distilled water | 500 [ml] |
| Compound Name | Dosage |
|---|---|
| chelate mix: 7% Fe; 1,3% B; 2% Mn; 0,06% Mo; 0,4% Zn; 0,1% Cu; EDTA; DTPA (ready-made products: TNC Trace, Plantex CSM+B, Microplex Chelating Agent-EDTA by Miller) | 10 [g] |
| ascorbic acid (E300) | 0,25 [g] |
| potassium sorbate (E202) | 0,1 [g] |
| warm distilled or RO water | 250 [ml] |
The method requires daily fertilizer dosing, so that the level of all nutrients is stable and to prevent any nutrient from being depleted.
Fertilizer should always be dosed at the same time of day, preferably just before the lighting is turned on.
Fertilizer dosing depends on:
Dosage for a 100% stocked aquarium (referring to the bottom surface of the aquarium), fairly intense lighting, where additional CO2 fertilizing is used:
We reduce the dose proportionately in case we: do not use additional CO2 fertilization, use weaker lighting, have fewer plants and do not perform partial water changes.
The indicator of whether the dosage used is correct will always be the condition of the plants in the aquarium, their growth rate (slow growth - too little or too much fertilizer), the appearance of algae (too much fertilizer), possible appearance of chlorosis on the leaves (too little fertilizer).
Fertilizer dosing can also be controlled by regularly testing for Fe (such a test must measure chelated iron) and NO3 (such a test must be accurate in the range of 5 mg/l). However, this is a troublesome, quite expensive and depending on the test used, an inaccurate method.
It may take some time to determine the correct and best fertilizer dosage for our plants, but it's worth the effort to later enjoy their healthy and beautiful appearance.
Such dosing allows the following nutrient concentrations to be maintained in the aquarium:
With this method, there are no stringent guidelines regarding partial water changes - other users of the method suggest making a 30-50% partial water change once a week or two.
As for additional CO2 fertilizing, its level in the aquarium should be stable, but not more than 20 ppm (20 mg/l).
Fertilizer in the PMDD method can be prepared on your own or bought ready-made. Before use, shake well and store in a dry, dark place at room temperature.
The PMDD method provides plants with a complete set of nutrients in relatively small amounts, ensures their moderate growth, and the limitation of phosphates means that we do not need such a large amount of carbon dioxide in the aquarium.
This method was created by Tom Barr and involves dosing nutrients (also phosphates) without the need to monitor these components in the aquarium (no need for tests). In this case, the fertilizer is dosed with a slight excess to ensure that the plants will not lack any component. This excess is removed at the beginning of each dosing cycle by conducting systematic, but large water changes to prevent possible overdose of the fertilizer (inhibition of its individual components).
The EI method allows to fertilize plants in an "approximate" amount, almost exactly required by them (estimating the amount of nutrients, not dosing a strictly defined dose).
This method is particularly recommended for aquariums with strong lighting and a large number of plants, but after appropriate dose reduction can also be used in tanks with weaker light.
| Compound Name | Dosage |
|---|---|
| potassium nitrate | 33 [g] |
| monopotassium phosphate | 7,2 [g] |
| magnesium sulfate | (*) |
| TNC GH Boost or Seachem Equilibrium | (**) |
| warm distilled or RO water | 250 [ml] |
(*) - add if your tap water is poor in magnesium – Mg < 5-10 mg/l;
(**) - add, if the total hardness of our water is very low, GH < 3.
| Compound Name | Dosage |
|---|---|
| chelate mix: 7% Fe; 1,3% B; 2% Mn; 0,06% Mo; 0,4% Zn; 0,1% Cu; EDTA; DTPA (ready-made preparations: TNC Trace, Plantex CSM+B, Microplex Chelating Agent-EDTA by Miller) | 10 [g] | ascorbic acid (E300) | 0,25 [g] |
| potassium sorbate (E202) | 0,1 [g] |
| warm distilled or RO water | 250 [ml] |
We can prepare fertilizer under the EI method ourselves or buy it ready-made. When it comes to ready-made fertilizers, we have two forms to choose from:
Fertilizer dosing cycle depends on:
| Day of the week | Activity |
|---|---|
| Monday | 50-70% water change 5 ml per 50 l of macronutrient fertilizer solution |
| Tuesday | 2,5 ml per 50 l of micronutrient fertilizer solution |
| Wednesday | 5 ml per 50 l of macronutrient fertilizer solution |
| Thursday | 2,5 ml per 50 l of micronutrient fertilizer solution |
| Friday | 5 ml per 50 l of macronutrient fertilizer solution |
| Saturday | 2,5 ml per 50 l of micronutrient fertilizer solution |
| Sunday | a day of rest |
Powder dosing depends on the aquarium volume and follows the same cycle as liquid fertilizer dosing.
| Day of the week | Activity | ||
|---|---|---|---|
| 40-80 [l] | 80-150 [l] | 150-225 [l] | |
| Monday | 50-70% water change 1/8 teaspoon KNO3 1/32 teaspoon KH2PO4 |
50-70% water change 1/4 teaspoon KNO3 1/16 teaspoon KH2PO4 |
50-70% water change 1/2 teaspoon KNO3 1/8 teaspoon KH2PO4 |
| Tuesday | 1/32 teaspoon of micronutrient fertilizer | 1/16 teaspoon of micronutrient fertilizer | 1/8 teaspoon of micronutrient fertilizer |
| Wednesday | 1/8 teaspoon KNO3 1/32 teaspoon KH2PO4 |
1/4 teaspoon KNO3 1/16 teaspoon KH2PO4 |
1/2 teaspoon KNO3 1/8 teaspoon KH2PO4 |
| Thursday | 1/32 teaspoon of micronutrient fertilizer | 1/16 teaspoon of micronutrient fertilizer | 1/8 teaspoon of micronutrient fertilizer |
| Friday | 1/8 teaspoon KNO3 1/32 teaspoon KH2PO4 |
1/4 teaspoon KNO3 1/16 teaspoon KH2PO4 |
1/2 teaspoon KNO3 1/8 teaspoon KH2PO4 |
| Saturday | 1/32 teaspoon of micronutrient fertilizer | 1/16 teaspoon of micronutrient fertilizer | 1/8 teaspoon of micronutrient fertilizer |
| Sunday | a day of rest | ||
The EI method requires daily fertilizer dosing, following a dosing cycle, so that the level of all nutrients remains stable to prevent exhaustion of any component.
The fertilizer should always be dosed at the same time of day, preferably just before switching on the light.
Fertilizer doses may be adjusted according to the needs of our plants. It is recommended to start dosing with a maximum dose and decrease it after completing a full three dosing cycles. We always make changes in the amount of fertilizer after a full three cycles. We continue to decrease the dose until we see undesired effects of too little fertilizer (poor plant condition) - then we increase the dose to the lowest level under which the plants were growing healthily (maintaining a three-week cycle).
The use of the EI method allows for maintaining the following nutrient concentrations in the aquarium:
When using this method, regular (weekly) and large (50-70%) partial water changes are required. This treatment is intended to remove any excess fertilizer and organic waste from the water.
When using this method, a high concentration of CO2 in the water is required and it must be kept at a constant level of 30 ppm (30 mg/l). The appearance of algae in the aquarium indicates that there is an inappropriate (too low) concentration of carbon dioxide in the water.
The use of the EI fertilization method also requires good water circulation in the aquarium - at least 10 times the nominal filter flow to the tank volume.
| Parameter | PMDD Method | EI Method |
|---|---|---|
| dosing |
|
|
| partial water changes |
|
|
| CO2 fertilization |
|
|
| lighting |
|
|
| use of tests |
|
|
| algae |
|
|
| types of aquariums |
|
|
Depending on the size of our aquarium, the type of lighting used in it, the quantity and type of plants as well as the number of fish and other aquatic animals, we can choose from one of three methods for fertilizing the water with carbon dioxide. These include:
The truth is that the advertised liquid CO2 preparations have nothing to do with gaseous carbon dioxide. These preparations are ordinary fertilizers enriching our water with organic carbon. They are not an alternative to carbon dioxide fertilization, but they support its use.
By itself, this type of fertilizer works well in small aquariums (up to 50 l), especially in shrimp tanks and tanks with a small amount of light (up to 0.6 W/l). They are also often used by aquarists who use high-pressure cylinders - as an additional source of carbon.
Advantages of using liquid preparations:
Disadvantages of using liquid preparations:
The homemade carbon dioxide fertilization system is called a homemade brew. The use of a homemade brew is recommended for medium-sized aquariums (up to 100 L) and lighting above 0.6 W/l.
The construction of a home brew is very simple - it consists of two plastic bottles connected with hoses. The operation of such a homebrew illustrates the diagram below:
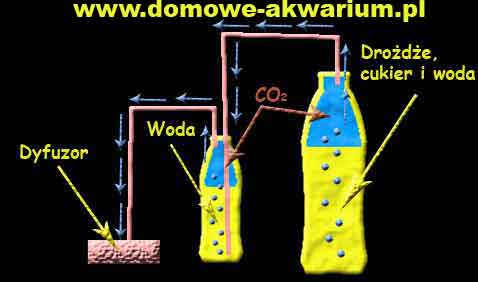
To prepare a homebrew, we will need two PET bottles with caps - 2 L (for the fermenting mash) and 0.5 L (for gas filtering/cleaning). We make a hole for the tube in the cap of the large bottle. We introduce the hose through it in such a way that it enters the bottle by about a centimeter (it cannot touch the mash). In the cap of the small bottle, we make two holes and place two tubes in them - one is the one that comes out of the large bottle and the other is the one that goes to the diffuser. The tube coming from the large bottle and entering the small one must be immersed in the water as low as possible. However, the tube going to the diffuser should be as high as possible, not touching the water. We seal the tubes in the caps with silicone so that carbon dioxide does not escape through leaks (silicone dries for about 24-48 h).
To prepare the mash we use 10 dag of culinary yeast, 400 g of sugar, and 1 L of lukewarm water. We dissolve the crushed yeast in the water. We do the same with the sugar - we put both ingredients in the 2 L bottle and pour the remaining water. Such a constructed homebrew will start supplying carbon dioxide in a short time (from 30 minutes to a few hours). You can increase or decrease the amount of yeast, which will give us longer operation with a lower intensity of CO2 or shorter operation with a stronger release of CO2. The operation time of the homebrew varies from 1 to 4 weeks.
You can use a very fine structure air stone as a diffuser - e.g. a linden wood cube.
At night, we should stop fertilizing with carbon dioxide, because in the darkness, photosynthesis processes do not occur - plants breathe with the oxygen produced during the day, and they excrete carbon dioxide into the water. It is easy then to exceed its concentration, and consequently to lower the pH (more carbonic acid in the water - more acidification of the water), and each change in pH by 1 degree actually results in a tenfold change in ion concentrations (logarithmic function).
Advantages of using homemade brew:
Disadvantages of using a homemade brew:
This method of enriching water with carbon dioxide is recommended for aquariums above 100 litres, which are strongly lit and have a preponderance of plants over animals or have demanding plant species.
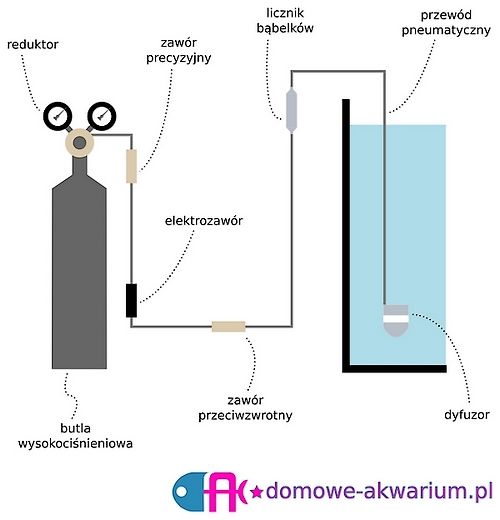
A basic set for CO2 fertilization using a high-pressure cylinder consists of:
Additional components to improve the kit's effectiveness are:
The set can be assembled using quick couplings (more convenient to use, but often cause leaks) or hard fittings (e.g.: nipples, sockets, etc.). The second method requires additional teflon tape or gasket sealant.
Additional accessories (gadgets) for the CO2 fertilization set
High pressure cylinder
Usually, cylinders used in aquaristics have a capacity of 0.5 kg or 2 kg. When choosing a bottle we should be guided by its current certifications (legalization), we should make sure that it is not regenerated e.g.: from an old extinguisher and whether it is equipped with a safety valve. An important parameter is also the possibility of refilling.
Reducer
Reducers are used to adapt the high pressure from the cylinder (reduce it) to a lower pressure used in aquaristics.
The reducers used in the CO2 fertilization kit can have 1 clock/pressure gauge (indicates the high pressure in the cylinder) or 2 clocks/pressure gauges (one indicates the high pressure in the bottle, the other the low pressure after reduction), may already be equipped with a precision needle valve, a non-return valve and/or a bubble counter - everything depends on the manufacturer and the purpose of the reducer (those intended for aquarium use generally have all the above-mentioned elements).
Pneumatic tube
A pneumatic tube is nothing more than a plastic hose used to connect all elements of our kit. It is important that it withstands high pressure.
Diffuser
The role of the diffuser is to facilitate the dissolution of carbon dioxide in water - breaking its bubbles into the smallest possible size (so-called microbubbles).
We distinguish between internal diffusers (placed inside the aquarium) and external (placed outside the aquarium - more effective).
Internal diffusers are open on one side, can be of various shapes (pipe-like, cylindrical, "flower"), made of plastic or glass, usually contain a ceramic sinter that breaks the gas bubbles, can be integrated additionally with a bubble counter (submerged spiral) or/and a non-return valve. The internal diffuser should be placed as close to the bottom as possible, under the outflow from the filter.
External (flow-through) diffusers are completely enclosed and also contain a ceramic sinter to break the bubbles. What distinguishes them from traditional diffusers is the possibility of direct connection to the outlet hose from an external filter or circulation pump. In this way, the water flowing through the diffuser saturates even 100% with carbon dioxide. Diffusers of this type are recommended primarily for very large aquariums.
Precision needle valve
It is used for precise carbon dioxide flow regulation.
It may be integrated with one of the set elements (e.g. reducer, diffuser) or you can purchase it separately and install it in an appropriate place on the pneumatic line.
Solenoid valve
It allows controlling the carbon dioxide fertilization - it allows adapting the method to the need of CO2 fertilization during the day and stopping fertilization at night. It is necessary to connect the solenoid valve to a timer (on/off switch) - the valve opens when connected to the power source (the valve is normally closed).
Depending on the construction of the solenoid it will heat up significantly (quite a big problem) or not. Solenoids that do not heat up during operation are impulse solenoids. Solenoids can be integrated with a non-return valve.
Non-return valve / return valve
This valve prevents backflow of water from the aquarium and protects our set from damage.
It may be integrated with one of the elements of our set (e.g. solenoid) or you can buy it separately and mount it in an appropriate place on the pneumatic tube.
Bubble counter
It allows controlling the carbon dioxide flow - with its help we can count bubbles in a unit of time.
It is usually a glass or plastic cylinder topped on both ends with suitable outlets for connection to the pneumatic tube.
The following diagram illustrates how to connect the kit:
As you know, an improper level of carbon dioxide carries serious consequences - too much of it rapidly lowers the pH of the water and causes diseases in fish (a disease caused by lack of oxygen). An overdose of CO2 is also bad for the condition of plants, as its presence in water affects the speed at which they absorb other nutrients. The more CO2, the faster and easier we can lead to macro and/or micro element deficiencies, and therefore their deficiency and poor plant condition. In this situation, algae outcompete plants for nutrients and quickly begin to dominate the aquarium.
That's why proper dosing of CO2 seems so important. Carbon dioxide in water is measured indirectly, based on the measurement of its pH. For this purpose we can use:
Carbon dioxide is present in water at a concentration similar to that in air, but in water, this gas dissolves about 10,000 times slower than in air. Its concentration is closely related to carbonate hardness and water pH. The higher the carbonate hardness, the smaller the pH and carbon dioxide fluctuations. The higher the water pH, the lower the CO2 concentration. Without additional CO2 fertilization, plants grow 6-10 times slower than when the gas is fertilized.
Additional carbon dioxide fertilization is not necessary in poorly lit aquariums with less demanding plants. But then we have to provide a few hours break in lighting for the CO2 absorbed by plants to regenerate (plants do not absorb carbon dioxide from water without light - on the contrary, they excrete it).
Additional carbon dioxide fertilization is necessary in strongly lit aquariums when we have a predominance of plants over aquatic animals. We also dose this gas when we grow submerged mud or terrestrial plants that grow completely emerged in their natural environment.
When we have the lighting turned off and it is dark, we never fertilize water with carbon dioxide - plants without light do not absorb CO2.
The amount of light and carbon dioxide is interrelated and it affects the speed at which plants absorb other nutrients. The less carbon dioxide in the aquarium, the less light we need.
With carbon dioxide fertilization, very good water circulation in the aquarium is recommended, but it is not recommended to use undergravel filters or air pumps - they accelerate the evaporation of gas from the tank.